WHO experts urge countries to continue using AstraZeneca coronavirus jab despite dangers
02/14/2022 / By Ramon Tomey
Experts from the World Health Organization (WHO) have recommended countries to continue using the AstraZeneca vaccine for treating the Wuhan coronavirus. They made the recommendation on March 17 after at least 10 nations suspended their use of the jab, following reports of post-vaccination adverse reactions. The global health body’s experts also added that they were looking into the vaccine’s available safety data in light of adverse reactions.
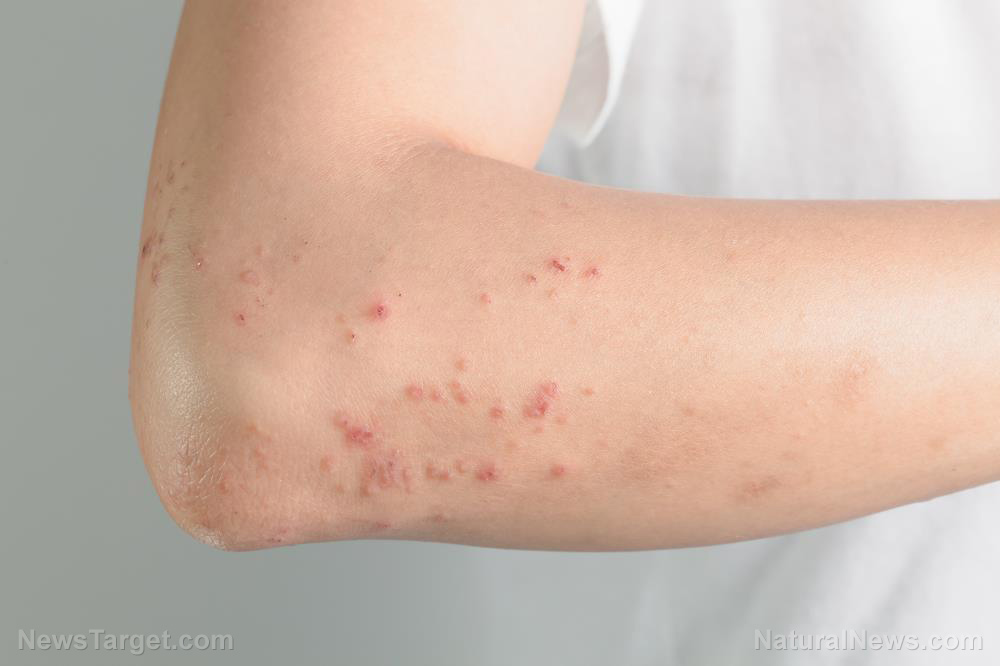
Reports of blood clots and brain hemorrhages in patients who received the AstraZeneca vaccine have derailed vaccination programs meant to address the pandemic. Most countries with vaccine doses from the British drug manufacturer suspended its use as a “precautionary measure.” WHO, European Medicines Agency (EMA) and AstraZeneca itself have insisted the vaccine is safe despite the purported links to thrombosis.
In a March 17 statement, vaccine experts from the global health body remarked it was still better for people to get the AstraZeneca vaccine than none at all. The statement said the WHO believes the vaccine’s benefits outweigh its risk, and recommended that ongoing vaccinations continue. The day before, WHO Director-General Tedros Adhanom Ghebreyesus said in a press conference: “This does not necessarily mean these [adverse] events are linked to the vaccine, but [it is] routine practice to investigate them – and it shows that the [vaccine] surveillance system works and effective controls are in place.”
The EMA concurred with the WHO’s recommendation to continue using AstraZeneca vaccines as it was not connected to the reported side effect. The European drug regulator said in a statement that it would hold a meeting on March 18 to finalize its conclusions and “make any necessary recommendations for further action. EMA Executive Director Emer Cooke meanwhile said during a March 16 press conference that “there is no indication that vaccination has caused these [serious] conditions.” She added: “A situation like this is not unexpected when you vaccinate millions of people.”
The AstraZeneca jab has been hounded with a myriad of issues from the beginning
AstraZeneca’s vaccine, manufactured in partnership with the University of Oxford, had been beset by various issues ever since its development stage. In September 2020, clinical trials for the jab were put on hold after two volunteers suffered from a serious adverse reaction. The following month, authorities in Brazil confirmed that one volunteer died during clinical trials there. The Oct. 15 death of the 28-year-old Rio de Janeiro resident was only reported to authorities four days later on Oct. 19.
In late November 2020, the vaccine came under fire after scientist-turned-writer Hilda Bastian pointed out the “shaky science” behind it. She wrote in a piece for Wired that a “dosing error” was responsible for purportedly higher efficacy rates for the jab. (Related: Aussie scientists cast doubt on low-efficacy AstraZeneca coronavirus vaccine.)
Trials conducted by AstraZeneca scientists were designed to test the effect of two full doses. However, some volunteers who got one and a half doses of the jab did not exhibit the usual high rate of adverse effects. Bastian elaborated: “[The] mistaken first half-dose, followed by a full dose at least a month later came in at 90 percent [efficacy], and the … two standard doses [given] at least a month apart achieved only 62 percent efficacy.”
But despite the issues surrounding the vaccine, some countries have opted to continue using the AstraZeneca vaccine.
The Philippines insisted on using the vaccine, having received more than 500,000 doses and administering 2.4 percent of these doses to its population. Presidential spokesman Harry Roque remarked that vaccinations in the Asian country will continue as “experts are saying … the benefits … are larger than the side effects of this vaccine.” He explained: “There is still no clear data that shows the blood clotting was caused by [the] AstraZeneca [jab.] If such data will come out, maybe we will also stop the [vaccine’s] use.”
Australia also expressed its continued use of AstraZeneca’s jab. Minister of Health and Aged Care Greg Hunt commented: “The government clearly, unequivocally [and] absolutely supports the AstraZeneca rollout. And the reason why is very simple – it will help save … and protect lives, and it’s done so on the basis of the medical advice.” Hunt received the British company’s COVID-19 vaccine alongside former Australian Prime Minister Julia Gillard, his post on Twitter said. (Related: Aussie Health Minister Greg Hunt hospitalized after getting the AstraZeneca coronavirus jab.)
Visit VaccineInjuryNews.com for more reports about the dangers of AstraZeneca’s COVID-19 vaccine.
Sources include:
Submit a correction >>
Tagged Under:
adverse reactions, AstraZeneca, Big Pharma, Blood clots, brain thrombosis, coronavirus vaccine, covid-19 pandemic, European Medicines Agency, pandemic, spike protein, vaccine injury, vaccines vaccine wars, World Health Organization, Wuhan coronavirus
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
Pandemic.News is a fact-based public education website published by Pandemic News Features, LLC.
All content copyright © 2018 by Pandemic News Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.